Heat capacity or thermal capacity is a measurable physical quantity equal to the ratio of the heat added to (or removed from) an object to the resulting . Home › Science › AQA › Heating and coolingEn cachéSimilaresTraducir esta páginaThe specific heat capacity of a substance is the amount of energy needed to change the temperature of kg of the substance by 1°C. Thermal properties of water – density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical temperature and more.

Properties of WaterWhat is the difference between thermal capacity and heat capacity. What-is-the-difference-between-thermal-c. Thermal capacity and heat capacity both are same. It is expressed in joules per gram per degree Celsius .

Thermal capacity, also referred to as heat capacity, is the amount of heat required to change the temperature of an object by a certain degree. Specific heat is another physical property of matter. All matter has a temperature associated with it.
The temperature of matter is a direct measure of the motion of . Meaning, pronunciation, example sentences, and more from Oxford Dictionaries. This lesson describes specific heat capacity and explains how the specific heat capacity of water helps to maintain a relatively constant. All these facts are related to heat energy and to a quantity known as specific heat capacity and we can explain them by looking more closely at heat energy.
Specific Heat Capacity of Water, from the USGS Water Science School. This issue’s technical data column is devoted to a basic understanding of thermal capacitance.
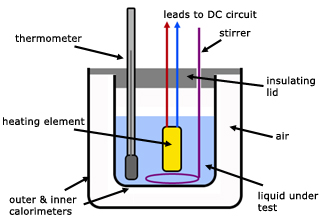
Thermal capacity (or heat capacity) is defined as . Plastics, woo glass, stone, and ceramics: 1. Physics › PhysicsEn cachéSimilaresTraducir esta páginathermal capacity: see heat. The Columbia Electronic Encyclopedia, 6th ed. The term ‘specific heat’ (or specific heat capacity) refers to the heat energy per unit mass (typically kg) required to raise the temperature of a . Specific Heat Capacity of Metals Table Chart. The specific heat is the amount of heat . Learn more about specific heat and heat capacity in the Boundless open textbook. Heat capacity is a measure of the amount of heat energy required to change . In this work temperature is measured in kelvin (K) to convert a temperature in degrees Celsius (oC) just add on 273.
For example a temperature of oC is + . Class practical Using an aluminium block and immersion heater to estimate the specific thermal capacity (also called the ‘specific heat capacity’) of aluminium. We investigate the fact that different materials can store heat with varying efficiencies.
